Covalent bond examples
Data on ionization energy (EI), PEI and the composition of stable molecules - their true values \u200b\u200band comparisons - of both free atoms and atoms bound in molecules, allow us to understand how atoms form molecules through the covalent mechanism ...
What is a single bond?
Lectures in Chemistry (Lecture) Eremin VV, Kargov S.I. Fundamentals of physical chemistry. Theory and Tasks (Document) Malinin N.N. Applied Theory of Plasticity and Creep (Document) OS Gabrielyan Chemistry. Grade 10. Basic Level (Document) Spurs in Chemistry ...
Big Encyclopedia of Oil and Gas
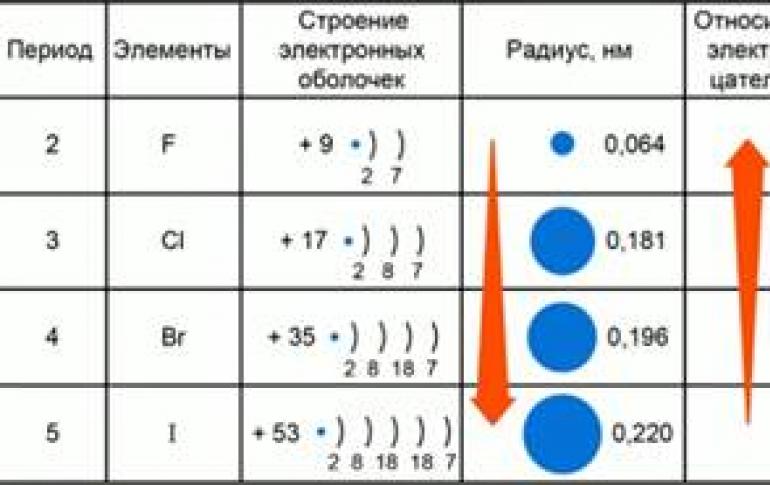
The concept of the radius of an atom and the electronegativity of elements, their dependence on the placement of elements in a periodic system Let us consider the relationship between the position of elements in a periodic system and such properties of chemical elements as ...
Simple (single) bond Types of bonds in bioorganic compounds
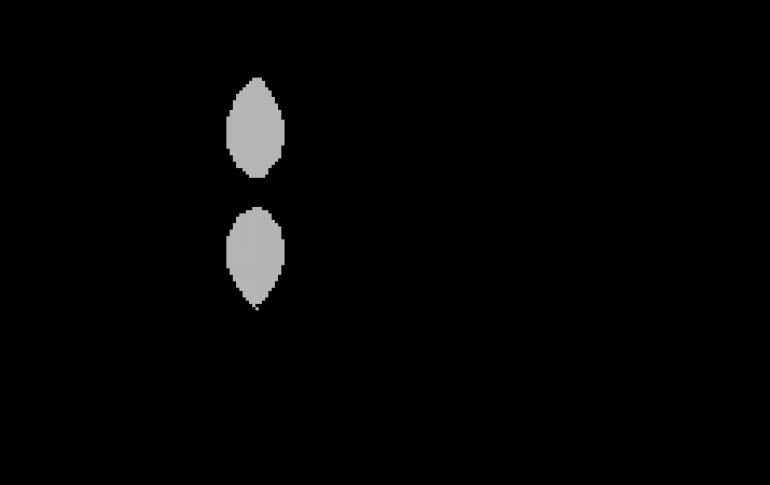
Double bond A covalent four-electron bond between two adjacent atoms in a molecule. D. s. it is usually indicated by two valence lines:\u003e C \u003d СC \u003d N ≈,\u003e С \u003d О,\u003e C \u003d S, ≈ N \u003d N ≈, ≈ Н \u003d О, etc. It is understood that one pair of electrons with ...
Communication mechanism
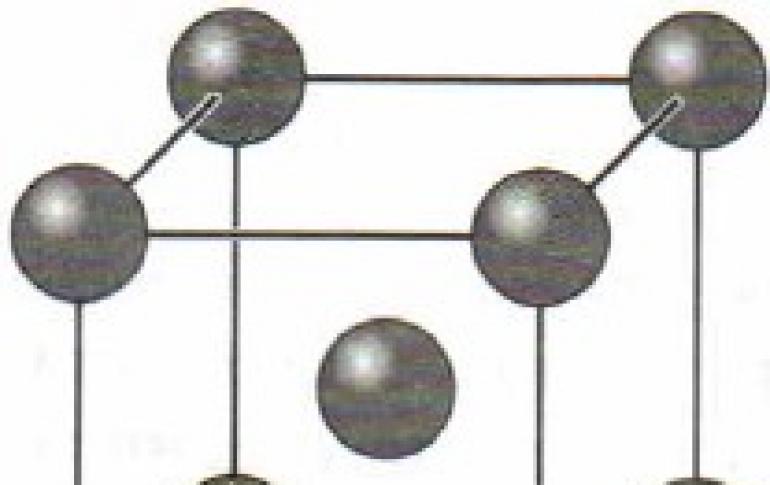
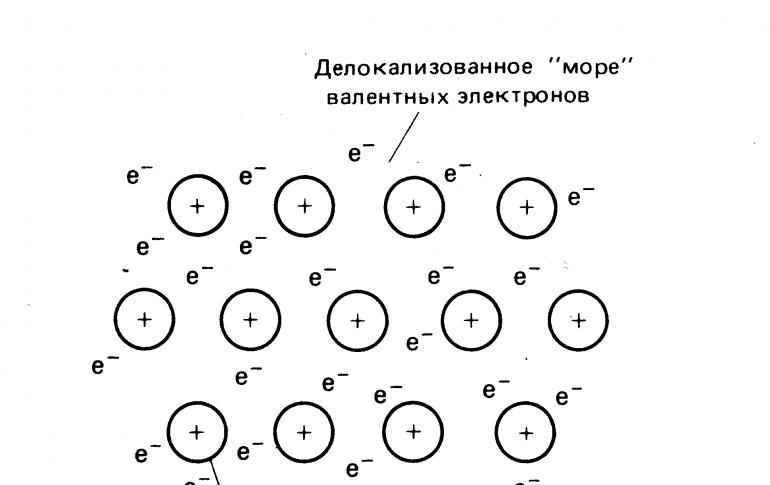
A metal bond is a bond formed between atoms under conditions of pronounced delocalization (the propagation of valence electrons through several chemical bonds in a compound) and a deficiency of electrons in an atom (crystal). Is an...
Chemical formula H2CO3 Type of molecule General information Carbonic acid weak dibasic acid
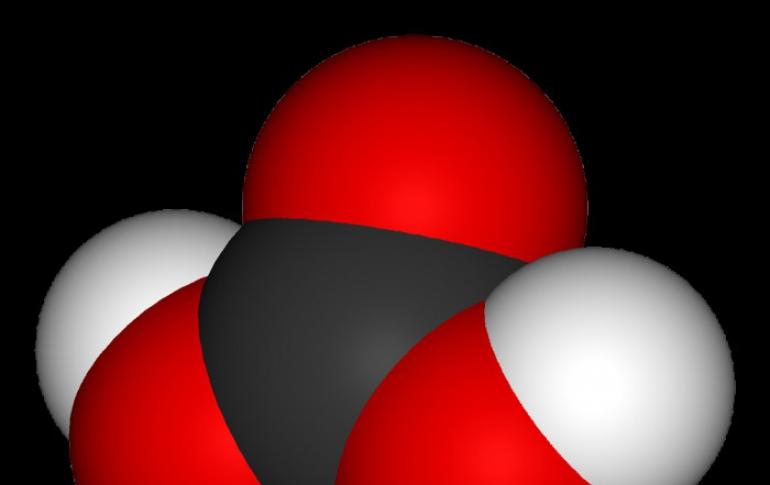
The more a person learns about the world around him, the more he realizes the limitations and imperfection of his knowledge. Take carbonated water, for example. As you know, this drink differs from others in that it is contained in small ...
The main types of chemical bonds

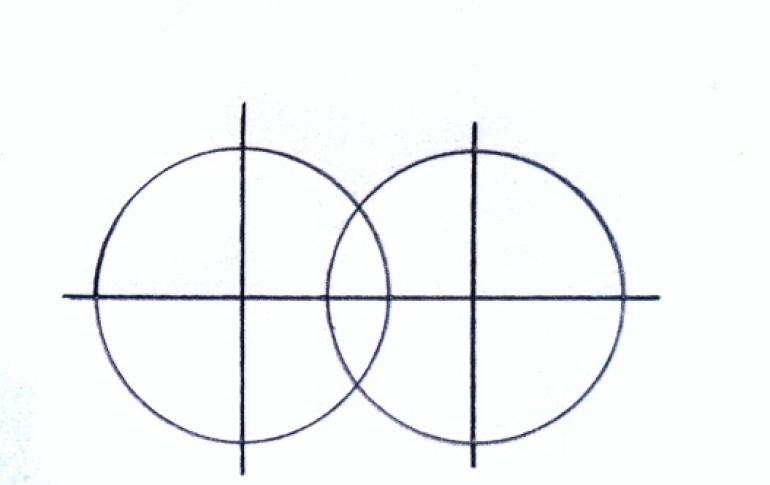
A covalent bond is a bond between two atoms due to the formation of a common electron pair. A covalent non-polar bond is a bond between atoms with equal electronegativity. For example: H 2, O 2, N 2, Cl 2, etc. Dipole moment ...
During the lessons, I watched the chalk leave its traces on the blackboard and I had a question: "Why do we write in chalk?

The poet’s work, the dialectic of the philosopher, the art of the researcher — these are the materials that make up the great scientist. Kliment Arkadyevich Timiryazev Kliment Arkadievich Timiryazev (06/03/1843–28.04.1920) - Russian natural scientist, physiologist –...
Connection of atoms among themselves
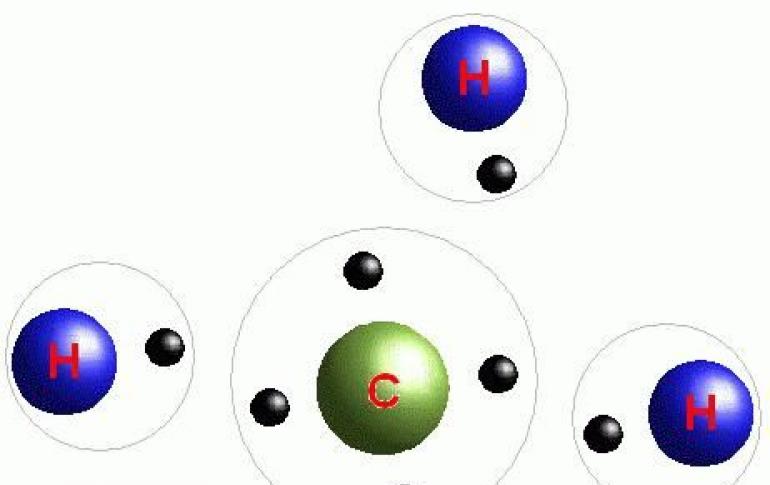
In accordance with the basic principle that matter always seeks to occupy the most favorable energy state, individual atoms have a more or less pronounced tendency to create an atomic compound. The difference in energy of an individual atom EA and ...
"Lysine Aescinat": instructions for use and recommended dosage
Complex edema of the soft tissues, poorly localizable, provoking point disturbances in the blood supply system and accompanied by pain, is a rather serious challenge for the pharmacological industry. Injuries of this kind more often ...


