Ionization energy data (EI), PEI and the composition of stable molecules are their real values \u200b\u200band comparisons - both free atoms and atoms associated with molecules, allow us to understand how atoms form molecules by means of a covalent bond mechanism.
Covalent communication - (from the Latin "CO" together and "VALES" with strength) (homeopolar communication), chemical communications Between two atoms arising from the hostility of electrons belonging to these atoms. Atoms in molecules of simple gases are connected by covalent bond. Communication at which there is one common pair of electrons is called single; There are also double and triple ties.
Consider several examples to see how we can use our rules to determine the number of covalent chemical bonds that may form an atom if we know the amount of electrons on the outer shell of this atom and the charge of its kernel. The charge of the nucleus and the amount of electrons on the outer shell are determined experimentally and are included in the element table.
Calculation of a possible number of covalent ties
For example, we calculate the number of covalent bonds that sodium may form ( Na)aluminum (AL),phosphorus (P),and chlorine ( CL). Sodium ( Na) and aluminum ( AL)they have, respectively, 1 and 3 electrons on the outer shell, and, according to the first rule (for the covalent communication mechanism, use one electron on the outer shell), they may form: sodium (NA) - 1 and aluminum ( AL) - 3 covalent bonds. After the formation of connections, the number of electrons on the outer shells of sodium ( Na) and aluminum ( AL) equals, respectively, 2 and 6; Those., less maximum number (8) for these atoms. Phosphorus ( P) and chlorine ( CL) They have, respectively, 5 and 7 electrons on the outer shell and, according to the second of the above-mentioned patterns, they could form 5 and 7 covalent bonds. In accordance with the fourth pattern, the formation of a covalent bond, the number of electrons on the outer shell of these atoms increases by 1. According to the sixth pattern, when a covalent bond is formed, the number of electrons on the outer shell of the binding atoms can not be more than 8. That is, phosphorus ( P) can form only 3 connections (8-5 \u003d 3), while chlorine ( CL) can form only one (8-7 \u003d 1).
Example: Based on the analysis, we found that some substance consists of sodium atoms. (NA) and chlorine ( CL). Knowing the patterns of the mechanism of forming covalent ties, we can say that sodium ( Na.) It can form only 1 covalent bond. Thus, we can assume that every sodium atom ( Na)associated with chlorine atom ( CL)by covalent bond in this substance, and that this substance consists of an atom molecules NaCl.. The formula of the structure for this molecule: Na - Cl. Here dash (-) means a covalent connection. The electronic formula of this molecule can be displayed as follows:
. .
NA: CL:
. .
In accordance with the electronic formula, on the outer shell of the sodium atom ( Na) in NaCl. There are 2 electrons, and on the outer sheath of the chlorine atom ( CL) There are 8 electrons. In this formula, electrons (points) between sodium atoms ( Na) and
chlorine (CL) are binding electrons. Since PEI in chlorine ( CL) equal to 13 eV, and sodium (NA) It is equal to 5.14 eV, the binder pair of electrons is much closer to the atom. Cl.than atom Na.. If the ionization energies of atoms forming the molecule differ greatly, then the resulting communication will polar covalent bond.
Consider another case. Based on the analysis, we found that some substance consists of aluminum atoms ( AL) and chlorine atoms ( CL). Aluminum ( AL) There are 3 electrons on the outer shell; Thus, it can form 3 covalent chemical bonds at that time chlorine (CL), as in the previous case, can form only 1 connection. This substance is represented as AlCl 3.and its electronic formula can be illustrated as follows:
Figure 3.1. Electronic formulaAlcl 3
whose formula of the structure:
Cl - Al - Cl
Cl.
This electronic formula shows that AlCl 3. on the outer sheath of chlorine atoms ( Cl.) There are 8 electrons, while on the outer sheath of the aluminum atom ( AL) Their 6. According to the mechanism for the formation of a covalent bond, both binders of an electron (one from each atom) come to the outer shells of binding atoms.
Multiple covalent bonds
Atoms having more than one electron on the outer shell can form not one, but several covalent bonds among themselves. Such connections are called multiple (more often multiple) Relations. Examples of such connections are the bonds of nitrogen molecules ( N.= N.) and oxygen ( O \u003d O.).
The connection formed by the union of single atoms is called homoatomic covalent tie, eif the atoms are different, the connection is called heteroatomic covalent tie [Greek prefects "Homo" and "Hetero" respectively mean the same and different].
Imagine, as in fact, it looks like a molecule with paired atoms. The simplest molecule with paired atoms is a hydrogen molecule.
7.8. Types of covalent bond
Covalent communication It is formed by overlapping electronic clouds of binding atoms. Exist different methods overlapping these electronic clouds.
1. Direct overlapping:
In this case, the only area of \u200b\u200boverlapping electron clouds lies on a straight line connecting the kernels of atoms. Communication formed in this way is called - Communication.
Depending on the type of overlapping clouds may form s-S. , s-P. , p-P. And other varieties of connection.
2. Side overlapping:

In this case, two areas of overlapping electron clouds are located on different directions from the plane in which the cores of the binding atoms lie. Communication formed in this overlapping EO is called a connection.
As in the case of a connection, depending on the type of overlapping clouds, various varieties of connection can be formed: p-P. , p-D. ,
d-D. etc.
And -, and -svyaz have a certain direction that occurs due to the desire of atoms to the maximum efficient overlapping of the EO, that is, to overlapping the clouds in the maximum electron density area. Thus, a covalent connection has a focus. For example, in the hydrogen sulfide molecule of H 2 S directions of two-beds between the sulfur atom and two hydrogen atoms are almost perpendicular (see the circuit on page 95). Atom, there is a completely defined number of unpaired electrons, so it can form a completely defined number of covalent ties. Thus, a covalent bond has saturation. For example, if a chlorine atom formed one-° C with a hydrogen atom (see the scheme on page 95), it can no longer connect with one hydrogen atom.
Comparison of characteristics - and -Cellies are shown in Table 20.
Table 20.Comparison of Characteristics - and - Communications
One overlapping area |
Two areas of overlapping |
Electronic clouds overlap with parts with the highest electron density |
Electronic clouds overlap with their peripheral parts |
Since it is almost always less durable, than -Cell, usually between atoms is first formed - α, and then, if there is an opportunity, then -cv. Consequently, it is possible only in the case of multiple ties (double and triple) formation:
| Cyanor garden - HCN. Other name - hydrocyanic acid. This is a colorless bat with a boiling point of 26 o C. With a strong heating or in the light it decomposes. Sinyl Acid is mixed with water in all respects. By analogy with halogen breeding, a solution of cyanovodorod in water is called cyanogenic acid. Sinyl Acid and its salts (cyanides) are very strong poisons (fatal dose for a person not more than 50 mg), and the acid itself can penetrate the body even through the intact skin. Once in the body, cyanode and cyanides are associated with hemoglobin in cyangemoglobin, affect the respiratory centers and cause choking. Despite its toxicity, syntic acid is used in the production of synthetic fibers and some types of plastics. In small concentrations, blue acid is found in the plant world (for example, in Gorky Almond). |
-Celm, -Svyaz.
1. The end of the paragraph shows the structural formulas of four substances. Make electronic and molecular formulas for them.
2.Sign the usual structural and electronic formulas of the following substances: CH 3 Cl, COF 2, SO 2 Cl 2 and N 2 H 4. In the case of difficulties, depict the formation of relations in these molecules. Specify B. structural formulas -And -Ovy. Keep in mind that in CH 3 CL atoms N and CL are associated only with atoms C, in COF 2 atoms O and F are also associated with carbon atoms, and in SO 2 Cl 2 atoms O and C1 are connected only with S. atoms.
7.9. Covalent bond energy
Communication strength is characterized by communication energy (see paragraph 7.5). The strength of the covalent bond can be estimated in two ways: determining the energy necessary for the breaking of all bonds in a certain portion of the substance, or by determining the energy necessary for the discontinuation of the known number of connections. In the first case, such an energy is called atomization energy, in the second - energy of communication. In practice, appropriate molar values \u200b\u200bare used.
The molar energy of atomization shows what energy should be spent on separation of 1 praying substance on insulated atoms.
The molar energy of communication shows which energy it is necessary to spend on the gap of 1 mole (6.02. 10 23) connections. For diatomic molecules, these energies coincide.
And the one, and the other molar energy is measured in kilodzhoules per mol: in the case of atomization energy - on the mol of the substance, and in the case of communication energy - on the mol of bonds. When calculating the number of links to determine the ES Dual (or triple), the connection is considered to be one bond.
Table 21.Examples of values \u200b\u200bE AT and average values \u200b\u200bof E SV (in KJ / Mol)
Substance |
Substance |
||||||
| H 2. | HF. | C- H. | N \u003d O. | ||||
| F 2. | HCL | N- H. | C- C. | ||||
| Cl 2. | HBR | O- H. | C \u003d C. | ||||
| Br 2 | HI | SI- H. | Cє C. | ||||
| I 2. | Co. | P- H. | Cє N. | ||||
| O 2. | IBR. | S- H. | SI-O. | ||||
| N 2. | CLF. | C \u003d O. | S \u003d O. |
From the values \u200b\u200bgiven in Table 21, it can be concluded that the strength of covalent bonds is the greater, the smaller the size of the binding atoms and more multiplicity of communication.
Molar atomization energy, molar communication energy.
7.10. The structure of molecules. Hybridization model
Most compounds with covalent bonds between atoms consists of molecules.
The concept of "structure of molecules" - a rather wide concept and includes, in particular, chemical structure and spatial structure.
The chemical structure of the molecule is described by the structural formula.
The spatial structure of the molecule is described by the spatial formula.
In order to characterize the spatial structure of the molecule quantitatively, it is necessary to determine the intelligent distances and the angles between the connections. Both can be determined experimentally.
To assess the interatomic distances in the molecules of substances, the spatial structure of which has not yet been studied, the so-called atomic (covalent) radii is often used.
The sum of atomic radii atoms of different elements is equal to the average distance between the atoms of these elements associated with a simple covalent bond, in molecules or crystals. The atomic radius table is shown in Appendix 9.
To estimate the corners between connections, a useful hybridization model is provided.
Recall the chemical structure of methane molecules (see Fig. On page 21). From the formation scheme of covalent bonds in this molecule (p. 105) it follows that three of the four connections in this molecule are exactly the same. Since the axis of electronic clouds P-AO is mutually perpendicular, then three covalent bonds formed with the participation of these clouds should be directed at right angles to each other. The fourth connection should differ from them somewhat. It is experimentally established that all four bonds in methane molecule are completely the same and sent in space as shown in the figure (p. 21). That is, carbon atom occupies a position in the center of the tetrahedron (the right tetrahedral, the triangular pyramid), and the hydrogen atoms in its vertices. This is possible only if the electronic clouds of the carbon atom involved in the formation of communication is absolutely the same and appropriately located in space.
As part of the hybridization model, it is assumed that such alignment really happens.
The hybridization of AO and EO is called hybrid.
In the case of methane CH 4 of hybridization, one 2S-AO and three 2p-JSC of carbon atom are subjected to, while four SP 3-hybrid JSC are formed. Schematically this can be written as:
1 (2S-AO) + 3 (2P-AO) 4 (SP 3 -AO).
The energies of orbitals become the same as the same: - Communications: To properly predict the structure of the molecule using the AO hybridization model, you must remember the following:
1) in the formation of covalent bonds at the atoms of elements of S- and P-blocks, which have only unpaired electrons (groups IIA, III and IVA), orbital, on which these electrons are always hybridized;
2) when the covalent bonds are formed by the atoms of the elements of the P-block, having an emergency pair (groups of VA and Via), hybridization is characteristic only for the atoms of the elements of the second period;
3) for the atoms of elements IA and VIIA groups, experimental confirmation of the presence or absence of hybridization is impossible;
4) if there are no obstacles, SP 3-hybridization is carried out; If there is not enough valence electrons for this, or some of them are involved in the formation of faces, then SP 2 - or SP-hybridization is carried out.
The chemical structure of the molecule, the spatial structure of the molecule, the interatomic distance, the angle between bonds, the atomic radius, hybridization of JSC, hybrid orbitals, the conditions of hybridization of JSC.
1. Increase the molecules of the following substances in order of increasing binding energy: a) H 2 S, H 2 O, H 2 TE, H 2 SE; b) PH 3, NH 3, SBH 3, ASH 3.
2. For the following molecules, draw the schemes for the formation of covalent bonds and determine the type of hybridization of central atoms AO: a) CCl 4, of 2, NF 3; b) bei 2, bf 3, siCl 4; c) H 3 C- CH 3, HCHO, N- with N.
Each atom consists of a positively charged kernel and a negatively charged electronic shell. Due to the charges of the kernel and electrons between adjacent atoms, electrostatic forces arise: attraction and repulsion. If the rapprochement of atoms leads to a decrease in the energy of the resulting particle (compared to the energies of individual atoms), a chemical bond is formed.
Chemical communications - These are the strengths of interaction, holding the particles of each other.
Scientists have proven that the main role in the formation of communication is played by electrons that are less associated with the nucleus, that is, located on the outer electronic shell. Such electrons are called valence.
In atoms of elements major subgroups All valence electrons are located on last (external) electronic layer and their number is equal to the group number.
In atoms of elements side subgroups Valence electrons are usually located on the last two electronic layers, But their number is also equal to the number of the group to which the element belongs.
For example, in the potassium atom, one valence electron, in the manganese atom, 7 valence electrons (Fig. 1).
![]()
Fig. 1. Electronic configurations of potassium and manganese atoms
According to the theory of chemical bond, the external shells of eight electrons are the most stable - octet (if in the atom only 1 electronic layer, then for it the most stable two-electron state is the doublet).
The formation of a stable e-shell can occur in several ways, therefore, different types of chemical bond distinguish.
Covalent communication - Chemical bond formed by overlapping electronic clouds of atoms. Electronic clouds (electrons), providing communication, are called a common electronic pair.
Two covalent bonding mechanisms are distinguished: exchange and donor-acceptor.
With the exchange mechanism, each atom provides one electron to form a common pair:
A · + B \u003d A: in
With a donor-acceptor mechanism, one atom provides a couple of electrons already existing ones (donor), and the other atom provides a free orbital for this pair of electrons (acceptor):
A: + □ B \u003d A: in
The relationship carried out by the formation of common electronic pairs, to the same extent belonging to both atoms, is called covalent non-polar.
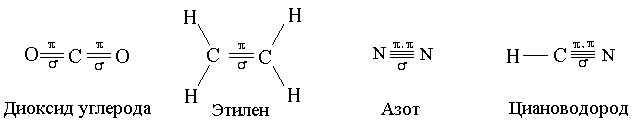
Covalent non-polar communication It is formed between the atoms of non-metals with the same values \u200b\u200bof relative electronegability, for example, in chlorine molecules, nitrogen, between carbon atoms in ethylene (Table 1).
|
Molecular formulas |
Electronic formulas |
Graphic formulas |
|
|
Table. 1. Examples of compounds in which covalent non-polar communications are present.
The number of common electronic pairs depends on how many electrons do not have enough each atom for the octet. Chlorine - element VII-A subgroup, therefore, on its outer electronic layer of electrons. The octet is not enough single electron, it means that one common pair of electrons in CL 2 will be formed. There are three common electronic pairs between nitrogen atoms in the n 2 molecule, that is, triple covalent bond. A double covalent bond is formed between carbon atoms in ethylene.
Please note that from each rule there are exceptions and the octet rule is not always performed (an example is a sulphous gas molecule SO 2).
Covalent polar communication It is carried out by the formation of general electronic pairs, which are shifted to an atom of a more electronegative element. In this case, partial charges are formed on atoms: δ + and δ- (Fig. 2).

Fig. 2. Education of a covalent bond in the chloride molecule
The greater the difference of electronegateness of atoms of elements, the greater the polarity of the communication.
Ion communication - limit case covalent polar communication.
Ion communication - This is an electrostatic attraction between ions formed by almost complete shift of the electronic pair to one of the atoms. This type of communication is formed if the difference of the values \u200b\u200bof the relative electronegability of atoms is large (as a rule, more than 1.7 on the genuine scale).
Ion communication usually formed between typical metaland typical nemetall. For example, in sodium chloride NaCl sodium atom 1 valence electron gave the chlorine atom and turned into a cation, and a chlorine atom, adopting 1 electron, turned into an anion. The anion cation is attracted, and an ion connection is formed (Fig. 3).
![]()
Fig. 3. Education of ion communication in sodium chloride
Salts, alkali, major oxides, carbides, nitrides belong to ionic connections. All these substances under normal conditions are solid, with high melting temperatures (usually 700-1000 ° C), their solutions and melts of electrically conduits.
The reflection of ionic compounds is explained by the fact that the ion can attract oppositely charged ions in any directions and large quantities. Consequently, the ions are firmly connected to the crystal lattice. For example, in a crystal sodium sodium grille, one sodium cation is surrounded by six chlorine anions, and each chlorine anion is surrounded by six sodium cations (Fig. 4). Thus, the entire crystal of the cooking salt is somehow a huge macromolecule consisting of a huge number of ions. AND chemical formula NaCl determines only their ratio in the crystal. Under normal conditions, the NaCl molecule does not exist.

Fig. 4. Model of the crystal sodium chloride lattice
In one substance, several types of chemical bond can be implemented. For example, in ammonium chloride there are covalent bonds formed in exchange and donor-acceptor mechanism, as well as an ionic connection between ammonium cation and chloride ion (Fig. 5).

Fig. 5. Education of chemical bonds in ammonium chloride
Summing up the lesson
You learned what a chemical connection is and why it is formed, what is the difference between the covalent and ionic relationship, how to portray the schemes of the formation of chemical bonds in various substances.
Bibliography
1. Novoshinsky I.I., Novoshinskaya N.S. Chemistry. Tutorial for 10 grade class. Creative Profile level. - M.: LLC "TID" Russian Word - RS ", 2008. (§§ 8, 14)
2. Kuznetsova N.E., Litvinova T.N., Lekun A.N. Chemistry: Grade 11: Textbook for students. Creative (Profile level): in 2 hours. M.: Ventana Graf, 2008. (§9)
3. Radetsky A.M. Chemistry. Didactic material. 10-11 classes. - M.: Enlightenment, 2011. (p. 88-95)
4. Homchenko I.D. Collection of tasks and exercises in chemistry for high school. - M.: RIA "New Wave": Publisher of Demolekov, 2008. (p. 39-41)
Homework
1.C. 39-40 Nos. 7.3, 7.5, 7.7, 7.17 of the collection of tasks and exercises in chemistry for high school (Khomchenko I.D.), 2008.
2. The list of substances: H 2 S, CO, KOH, K 2 O, NA 2 SO 4, CUCl 2, Hi, S, PCl 3, N 2 O 5. Write out of the formulas of substances from it: a) with ion bond; b) with a covalent bond.
3. Make an electronic formula of the SO 2 molecule. Show electronic density offset. Specify the type of chemical bond.
I first explained the structure of the electronic shell, contributed to the creation of the idea of \u200b\u200bthe chemical bond and its electronic nature. In accordance with the BOR model, electrons can occupy in the atom of the position, which correspond to certain energy states, i.e. energy levels. In 1915 The German physicist Kossel gave an explanation of the chemical bonds in the salts, and in 1916, the American scientist Lewis proposed the interpretation of the chemical bond in molecules. They proceeded from the ideas that the atoms of the elements have a tendency to achieve electronic configuration of noble gases (full filling of the outer electronic layer). The representations of Kossel and Lewis received the names of the electronic theory of valence.
Valinity of elements of the main subgroups Periodic system Depends on the number of electrons located on the outer electronic layer. Therefore, these external electrons are called valence. For elements of side subgroups, both the electrons of the outer layer and the electrons of the internal sublevel can appear as valence electrons.
There are three main types of chemical bonds: covalent, ionic, metallic.
Table. Types of chemical bonds and their main distinguishing features.
| Chemical communications | Binding atoms | Character of elements | Process in electronic shell | Parts formed | Crystal cell | Industrial character | Examples |
| Ionic | Metal atom and atom Nemetalla | Electropolo- Living I. electrical negative |
Transition of valence electrons | Positive and negative ions | Ionic | Saline nyu |
NaCl Cao Naoh. |
| Covalent | Nemmetalov atoms (less often atoms of metals) | Electrical alarmer Living |
Education of common electronic pairs, filling molecular orbitals | Molecules |
Molecular |
Fly or non-volatile | BR 2 CO 2 C 6 H 6 |
| --------- | Atomic | Almond-like nyu |
Diamond Si Sic | ||||
| Metal Kaya. |
Atoms of metals | Electropolo- Living |
Return of valence electrons | Positive ions and electronic gas | Metal | Metal- Kaya. |
Metals and alloys |
Covalent connection.
Covalent bond is formed due to general electronic pairs arising in the shells of associated atoms.
It is necessary to introduce the concept of electronegativity. Electricity is the ability of atoms chemical element Press the general electronic pairs involved in the formation of a chemical connection.

A number of electronegateness
Relative electronegability elements (by poling)
| group | I. | II. | III | IV | V. | VI | VII | VIII. | |||
| period | |||||||||||
| 1 | H. 2,1 |
He. - |
|||||||||
| 2 | LI 0,97 |
BE. 1,47 |
B. 2,01 |
C. 2,50 |
N. 3,07 |
O. 3,5 |
F. 4,10 |
Ne - |
|||
| 3 | Na. 1,01 |
MG. 1,23 |
Al 1,47 |
SI 1,74 |
P. 2,1 |
S. 2,6 |
Cl. 2,83 |
AR - |
|||
| 4 | K. 0,91 |
CA. 1,04 |
SC 1,20 |
TI 1,32 |
V. 1,45 |
CR 1,56 |
MN. 1,60 |
FE. 1,64 |
Co. 1,70 |
Ni. 1,75 |
|
| Cu. 1,75 |
Zn. 1,66 |
GA. 1,82 |
GE. 2,02 |
As 2,20 |
SE 2,48 |
Br. 2,74 |
Kr. - |
||||
| 5 | RB. 0,89 |
Sr. 0,99 |
Y. 1,11 |
Zr. 1,22 |
NB. 1,23 |
Mo. 1,30 |
TC. 1,36 |
Ru 1,42 |
Rh. 1,45 |
Pd. 1,35 |
|
| AG 1,42 |
CD 1,46 |
IN. 1,49 |
SN. 1,72 |
SB. 1,82 |
TE 2,01 |
I. 2,21 |
Xe. - |
||||
| 6 | CS. 0,86 |
BA. 0,97 |
LA * 1,08 |
HF. 1,23 |
TA. 1,33 |
W. 1,40 |
Re. 1,46 |
OS 1,52 |
IR. 1,55 |
Pt. 1,44 |
|
| AU. 1,42 |
Hg. 1,44 |
TL 1,44 |
PB. 1,55 |
BI 1,67 |
PO 1,76 |
AT. 1,90 |
RN. - |
||||
| 7 | Fr. 0,86 |
RA 0,97 |
AC ** 1,00 |
* Lantanoids - 1.08 - 1.14 | |||||||
Rarely chemical substances Consist of separate, not related atoms of chemical elements. In such a building, only a small number of gases called noble: helium, Neon, Argon, Krypton, Xenon and Radon have such a structure. More often, chemicals are not consisting of disparate atoms, but from their associations to various groups. Such integration of atoms can withdraw several units, hundreds, thousands or even more atoms. The force that keeps these atoms as part of such groups is called chemical communications.
In other words, it can be said that the chemical bond is called interaction, which provides the relationship of individual atoms into more complex structures (molecules, ions, radicals, crystals, etc.).
The reason for the formation of a chemical bond is that the energy of more complex structures is less than the total energy of individual, forming it atoms.
So, in particular, if the XY molecule is formed in the interaction of x and y atoms, this means that the internal energy of the molecules of this substance is lower than the internal energy of individual atoms, of which it was formed:
E (XY)< E(X) + E(Y)
For this reason, in the formation of chemical bonds between individual atoms, energy will be allocated.
In the formation of chemical bonds, the electrons of the external electronic layer with the smallest communication energy with the kernel are involved, called valentines. For example, the bora has electrons 2 of the energy level - 2 electrons on 2 s-orbital and 1 to 2 p.-theliti:
In the formation of a chemical bond, each atom seeks to obtain an electronic configuration of noble gases atoms, i.e. So that in its outer electron layer there are 8 electrons (2 for the first period elements). This phenomenon received the name of the octet rule.
The achievement of electronic configuration atoms of noble gas is possible if initially single atoms will make part of their valence electrons by common for other atoms. At the same time, general electronic pairs are formed.
Depending on the degree of electron coercion, covalent, ionic and metallic communications can be distinguished.
Covalent communication
Covalent bond occurs most often between the atoms of non-metal elements. If non-metal atoms forming a covalent bond belong to different chemical elements, such a connection is called covalent polar. The reason for such a name lies in the fact that atoms of different elements have different ability to attract a common electronic pair to themselves. It is obvious that this leads to a displacement of a common electron pair towards one of the atoms, as a result of which a partial negative charge is formed on it. In turn, a partial positive charge is formed on another atom. For example, in the molecule of chloroodor electronic para shifted from hydrogen atom to the chlorine atom:
Examples of substances with a covalent polar bond:
CCl 4, H 2 S, CO 2, pH 3, SiO 2, etc.
Covenate non-polar connection is formed between the atoms of non-metals of one chemical element. Since atoms are identical, the same and their ability to delay general electrons. In this regard, the displacement of the electronic pair is not observed:
The above-described covalent bond formation mechanism, when both atoms provide electrons for the formation of general electronic pairs, is called exchange rate.
There is also a donor-acceptor mechanism.
In the formation of a covalent bond on the donor-acceptor mechanism, the general electron pair is formed due to the orbital of one atom (with two electrons) and the empty orbital of the other atom. An atom providing a watery electron pair is called a donor, and an atom with a free orbital - acceptor. An atoms have paired electrons, for example N, O, P, S.
For example, according to the donor-acceptor mechanism, the fourth covalent n-H communication In the ammonium cation NH 4 +:
In addition to polarity, covalent bonds are also characterized by energy. Communication energy is called minimal energy necessary to break the bond between atoms.
Communication energy decreases with increasing radii of binding atoms. As we know atomic radii Increases down the subgroups, it is possible, for example, to conclude that halogen-hydrogen bond strength increases in a row:
HI< HBr < HCl < HF
Also, the binding energy depends on its multiplicity - the greater the multiplicity of communication, the greater its energy. Under the multiplicity of communication is understood as the number of general electronic pairs between two atoms.
Ion communication
Ionic communication can be viewed as an extreme case of covalent polar communication. If a general electron pair is displaced in a covalent and polar connection to one of the pair of atoms, then in the ionic it is almost completely "given" one of the atoms. An atom who gave an electron (s) acquires a positive charge and becomes cation, and an atom who climbed his electrons, acquires a negative charge and becomes anion.
Thus, the ion connection is a relationship formed by electrostatic attraction of cations to anions.
The formation of this type of communication is characteristic of the interaction of typical metals and typical non-metals.
For example, potassium fluoride. Potassium cation is obtained as a result of the separation from the neutral atom of one electron, and the fluorine ion is formed when the fluorine is connected to the one electron atom:

A power of electrostatic attraction arises between the resulting ions, as a result of which the ionic connection is formed.
In the formation of chemical bonds, electrons from the sodium atom moved to the chlorine atom and the oppositely charged ions were formed, which have a complete external energy level.
It has been established that the electrons from the metal atom do not extend completely, but only shift towards the chlorine atom, as in a covalent bond.
Most binary compounds that contain metal atoms are ionic. For example, oxides, halides, sulphides, nitrides.
The ion connection also occurs between the simple cations and simple anions (F -, CL -, S 2-), as well as between simple cations and complex anions (NO 3 -, SO 4 2-, PO 4 3-, OH -). Therefore, ionic compounds include salts and bases (Na 2 SO 4, Cu (NO 3) 2, (NH 4) 2 SO 4), Ca (OH) 2, NaOH)
Metal communication
This type of communication is formed in metals.
At the atoms of all metals on the outer electron layer there are electrons that have low bond energy with the atomic core. For most metals, the process of losing external electrons is energetically beneficial.
In view of such a weak interaction with the nucleus, these electrons in metals are very mobile and in each metal crystal continuously occurs the following process:
M 0 - ne - \u003d m n +,
where m 0 is a neutral metal atom, and M n + the cation of the same metal. The figure below shows the illustration of the processes occurring.
That is, the electrons are "used" by the metal crystal, disconnecting from one metal atom, forming a cation from it, connecting to another cation, forming a neutral atom. Such a phenomenon was called "Electronic Wind", and the combination of free electrons in the crystal of the Nemmetall atom was called "Electronic Gas". A similar type of interaction between atoms of metals was called a metal tie.
Hydrogen communications
If a hydrogen atom in any substance is associated with a high electrone element (nitrogen, oxygen or fluorine), such a phenomenon is characterized as a hydrogen bond.
Since the hydrogen atom is associated with an electronegative atom, a partial positive charge is formed on the hydrogen atom, and on the atom of the electronegative element - partial negative. In this connection, it becomes possible to electrostatic attraction between a partially positively charged hydrogen atom of one molecule and an electro-negative atom of another. For example, hydrogen bond is observed for water molecules:
It is a hydrogen bond that explains abnormally heat Melting water. In addition to water, also durable hydrogen bonds They are formed in such substances as fluoride hydrogen, ammonia, oxygen-containing acids, phenols, alcohols, amines.



