Electricity is called the property of the chemical element to attract the electrons to its atom from atoms of other elements with which this element forms a chemical bond in connections.
When the chemical bond is formed between atoms of different elements, the general electronic cloud shifts to a more electrone-negative atom, which is why the connection becomes covalently polar, and with a large difference of electronegateness - ion.
Electricity is taken into account when writing chemical formulas: the symbol of the most electronegative element is recorded in the binary connections.
Electricity increases in the direction from left to right for elements of each period and decreases in the direction from top to bottom for the elements of the same PS group.
Valence The element is called the property of its atoms to be connected to a certain number of other atoms.
There are stoichiometric, electronic valence and coordination number. We will consider only stoichiometric valence.
Stoichiometric Valence shows how many atoms of another element attachs an atom of this element. Per unit of valence adopted valence of hydrogen, because Hydrogen is always monovalent. For example, in HCl, H 2 O, NH 3 compounds (proper writing of ammonia H 3 n is already used in modern manuals), CH 4 chlorine is monovalent, oxygen bivalent, nitrogen trivalent and carbon tetravalenten.
The stoichiometric valence of oxygen is usually equal to 2. Since almost all elements form compounds with oxygen, it is convenient to use it as a reference to determine the valence of another element. For example, in compounds Na 2 O, Coo, Fe 2 O 3, SO 3 sodium monovalent, cobalt bivalent, iron is trivalent, sulfur hexavalent.
In oxidative and restorative reactions, it will be important for us to determine the degrees of elements oxidation.
Degree of oxidation The element in the substance is called its stoichiometric valence, taken with a plus sign or minus.
Chemical elements are divided into elements permanent valence Valence variable elements.
1.3.3. Molecular and non-elastic substances. Type of crystal lattice. The dependence of the properties of substances from their composition and structure.
Depending on which state of the compound is in nature, they are divided into molecular and non-enecular. IN molecular substances The smallest structural particles are molecules. These substances have a molecular crystal lattice. In non-mecular substances, atoms or ions are the smallest structural particles. The crystal lattice is atomic, ion or metal.
The type of crystal lattice largely determines the properties of substances. For example, metals having metal type of crystal latticediffer from all other elements high plasticity, electric and thermal conductivity. These properties, as well as many others - forging, metal gloss, etc. due to a special type of communication between metal atoms - metal connection. It should be noted that the properties inherent in metals are manifested only in condensed state. For example, silver in a gaseous state does not have the physical properties of metals.
A special type of communication in metals is metallic - due to the deficiency of valence electrons, so they are common to the entire structure of the metal. The most simple model of the metal structure assumed that the crystalline lattice of metals consists of positive ions surrounded by free electrons, the movement of electrons occurs chaotic, like gas molecules. However, such a model, a qualitatively explaining many properties of metals, with a quantitative check turns out to be insufficient. Further development of the theory of the metal state led to the creation zone theory of metalswhich is based on the performances of quantum mechanics.
In the nodes of the crystal lattice there are cations and metal atoms, and the electrons are freely moved along the crystal lattice.
The characteristic mechanical property of metals is plasticdue to the peculiarities of the inner structure of their crystals. Under the plasticity, the ability of bodies under the action of external forces to undergo deformation, which remains and after the cessation of external influence. This property of metals allows them to give them a different form when cutting, ride metal into sheets or pull it into a wire.
The plasticity of metals is due to the fact that with the external effects of layers of ions forming the crystal lattice, shifted relative to each other without breaking. This occurs as a result of the fact that moving electrons due to free redistribution continues to communicate between ion layers. In the mechanical effect on the solid with a nuclear grille, its separate layers are shifted and the adhesion between them is broken due to the rupture covalent ties.
ionsthen these substances form ion type of crystal lattice.
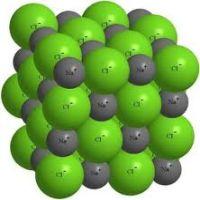
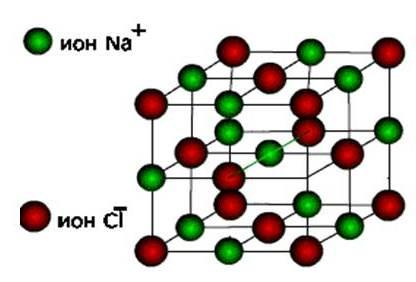
These are salts, as well as oxides and hydroxides of typical metals. These are solid, fragile substances, but their main quality : Solutions and melts of these compounds conduct electric current.
If in the nodes of the crystal lattice are located atomsthen these substances form atomic type of crystal lattice(Diamond, Bor, silicon aluminum and silicon oxides). According to the properties, very solid and refractory, insoluble in water.
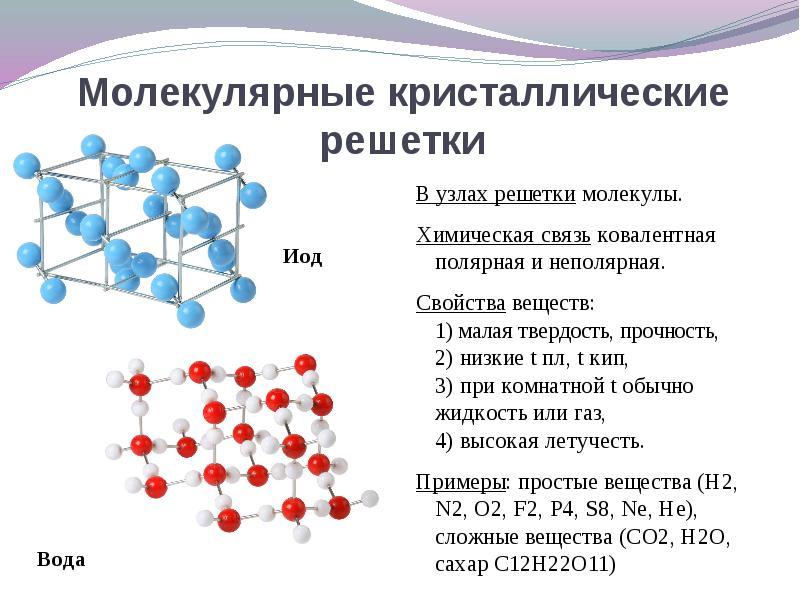
If in the nodes of the crystal lattice are located moleculesThese substances form (under normal conditions of gases and liquids: O 2, HCl; I 2 organic substances).
It is interesting to note the metal of gallium, which melts at a temperature of 30 o C. This anomaly is explained by the fact that Ga 2 molecules and its properties are located in the assemblies of the crystal lattice.
Example.Nemolecular structure have all non-metals of the group:
1) carbon, boron, silicon; 2) fluorine, bromine, iodine;
3) oxygen, sulfur, nitrogen; 4) chlorine, phosphorus, selenium.
IN non-elastic substances The smallest structural particles are atoms or ions. The crystal lattice is atomic, ionic or metal
For solution This question is easier to go from the opposite. If in the nodes of the crystal lattice are located moleculesthen these substances form molecular type of crystal lattice(under normal conditions of gases and liquids: O 2, HCl; also i 2, rhombic sulfur S 8, white phosphorus P 4, organic substances). By properties, these are fragile low-melting compounds.
In the second answer there is a fluorine gas, in the third - gases oxygen, nitrogen, in the fourth - chlorine gas. So these substances have a molecular crystal lattice and a molecular structure.
IN first Answer all substances - solid compounds under normal conditions and form a nuclear grille, which means that there are a non-ethics.
Correct answer:1) carbon, Bor, Silicon
- The least electronegative is the chemical element.
- iron
- magnesium
- calcium
Attention should be paid to the phrase "least electronegative", i.e. the element with the largest metal properties. This argument will eliminate from possible Azot responses as non-metall, and to dwell on calcium, as the most active of the metals proposed in the task. Answer: 4.
- The most polar chemical bond in one of the molecules
- CL 4.
- St. 4.
Knowledge of the patterns of changes in electron monateness in periods and groups of the periodic system D. I. Mendeleev makes it possible to exclude from the list of quadricular compounds of carbon methane CH 4, and from the remaining halides to stop at CF 4, as on the compound of carbon with the most electronegative of all chemical elements - fluorine. Answer: 2
- In chloride molecules and chlorine chemical communication, respectively
- ionic and covalent polar
- ionic and covalent non-polar
- covalent polar and covalent non-polar
- hydrogen and covalent non-polar
The keyword for the quick and correct execution of this task is the word "respectively". In the proposed versions, only one of the answers begins with the words "covalent polar", i.e. the bonds characteristic of chloroodor. Answer: 3.
- The degree of oxidation of manganese in the compound, the formula of which to 2 MnO 4, is equal
Knowledge of the rules for calculating the degrees of oxidation of elements by the formula will allow you to choose the right answer. Answer: 3.
- The smallest degree of oxidation has a sulfur in salt
- sulfate potassium
- sulfit potassium
- sulfide potassium
- potassium hydrosulfate
Obviously, the rapid fulfillment of this task will be to translate the names of the salts in the formula. Since sulfur is an element of the group VIA, then its smallest degree of oxidation is -2. This value corresponds to a compound with the formula K 2 S - potassium sulfide. Answer: 3.
- The degree of oxidation is +5 chlorine atom has in ion
- C1O - 4.
- C1O -
- C1O - 3.
- C1O - 2.
When performing this task, you should pay attention to the fact that there are no electronic compounds in the condition, but chlorine ions with a single negative charge ("-"). Since the sum of the degrees of the oxidation of atoms in the ion is equal to the charge of the ion, the total negative charge of the oxygen atoms in the articulated ion should be -6 (+5 - 6 \u003d -1). Answer: 3.
- The degree of oxidation -3 nitrogen has in each of the two connections
- NF 3 and NH 3
- NH 4 Cl and N 2 O 3
- NH 4 Cl and NH 3
- HNO 2 and NF 3
To determine the right response, it is necessary to mentally divide the options for answers to the left and right beep. Then choose the one in which the compounds have a simpler composition - in our case, this is the right bented binary compound. The analysis will eliminate the responses 2 and 4, as in oxide and fluoride in nitrogen the positive degree of oxidation, as in a less electronegative element. This argument makes it possible to exclude and respond 1, since it is the first substance - all the same nitrogen fluoride. Answer: 3.
- To the substances of the molecular structure do not include
- carbon dioxide
- methane
- chloroorod
- calcium carbonate
You should pay attention to the negative judgment, laid down in the assignment condition. Since the gaseous substances under normal conditions have a molecular crystalline grille in a solid state, the assignment condition 1-3 does not correspond to the condition condition. Calcium carbonate attribution to the salts again confirms the right answer. Answer: 4.
- Are the following judgments about the properties of substances and their structure?
A. Wet underwear dries in frost because the molecular structure substances are capable of sublimation (sublimation).
B. Wet underwear dries in frost because water molecules have a low molecular weight.
- true only A.
- right only B.
- both judgments are true
- both judgments are not true.
Knowledge physical properties The substances of the molecular structure makes it possible to decide that the cause of the dry laundry drying in the frost is the ability of ice to sublimation, and not the dipole structure of water molecules. Answer: 1.
- The molecular structure has each of the substances, the formulas of which are given in the row
- CO 2, HNO 3, SAO
- Na 2 S, Br 2, NO 2
- H 2 SO 4, Cu, O 3
- SO 2, I 2, NSL
Since the proposed options contain three substances, it is logical to mentally divide these versions into three vertical beeps. The analysis of each of them, starting with substances of a simpler composition (average podstolbik), will eliminate the response 3, since it contains metal copper, having a metal crystalline grid. A similar analysis of the right beep will make it possible to eliminate the response 1, as it contains the oxide of the pitch-earth metal (ion lattice). Of the two remaining options, it is necessary to exclude the option 2, since it contains an alkali metal salt - sodium sulphide (ion lattice). Answer: 4.
Tasks for independent work
- The degree of oxidation is +5 nitrogen exhibits in the compound, the formula of which
- N 2 O 5
- N 2 O 4
- N 2 O.
- The degree of oxidation of chromium in the compound, the formula of which (NH 4) 2 Cr 2 O 7, is equal to
- The degree of oxidation of nitrogen decreases in a row of substances, the formulas of which
- NH 3, NO 2, KNO 3
- N 2 O 4, KNO 2, NH 4 CL
- N 2, N 2 O, NH 3
- HNO 3, HNO 2, NO 2
- The degree of oxidation of chlorine increases in a number of substances, the formulas
- NSLO, NSLO 4, KSLO 3
- CL 2, C1 2 O 7, KSLO 3
- CA (C1O) 2, KSLO 3, NSLO 4
- KSL, KSLO 3, KSLO
- The most polar chemical communication in the molecule
- ammonia
- serovodorod.
- bromomodorod
- fluorodorod
- Substance with covalent non-polar bond
- white phosphorus
- aluminum phosphide
- phosphorus chloride (V)
- calcium phosphate
- Formulas of substances only with ion bond recorded in a row
- sodium chloride, phosphorus chloride (V), sodium phosphate
- sodium oxide, sodium hydroxide, sodium peroxide
- seroublerod, calcium carbide, calcium oxide
- calcium fluoride, calcium oxide, calcium chloride
- Atomic crystal lattice has
- sodium oxide
- calcium oxide
- sulfur oxide (IV)
- aluminium oxide
- Connection with ionic crystal lattice formed when the chlorine interacts with
- phosphorus
- barium
- hydrogen
- gray
- Are the following judgments about Ammonium chloride?
A. Ammonium chloride is the ionic structure substance formed by covalent polar and ionic ties.
B. Ammonium chloride - the substance of the ion structure, and therefore solid, refractory and non-volatile.
- true only A.
- right only B.
- both judgments are true
- both judgments are incorrect
08. Electricity, oxidation degree, oxidation and recovery
Let's discuss the meaning of extremely interesting concepts that exist in chemistry, and as often happens in science, sufficiently confusing, and used in the inverted form. It will be about "electronegativity", "the degree of oxidation" and "redox reactions".
What does this mean - the concept is used in an inverted form?
We will try to gradually tell about it.
Electricity Demonstrates us the redox properties of the chemical element. That is, his ability to take or give free photons. And is it also a source or energy absorber (ether). Yang or Yin.
Degree of oxidation - This is a concept similar to the concept of "electronegativity". It also characterizes the redox properties of the element. But between them there is the next difference.
Electricity gives the characteristic of a separate element. By itself, out of finding it in a chemical compound. While the degree of oxidation characterizes its oxidative and restorative abilities precisely when the element is part of any molecule.
Let's talk a little about what is the ability to oxidize, and what is the ability to restore.
Oxidation - This is the process of transferring to another element of free photons (electrons). Oxidation is not excluding electrons at all, as is now considered to be in science. . When the element oxidizes another element, it acts like an acid or oxygen (hence the name "oxidation"). Oxidize - it means to promote destruction, decay, burning elements . The ability to oxidize is the ability to cause the destruction of molecules by the energy transmitted by them (free photons). Remember that energy always destroys the substance.
Surprisingly, how long in science there are contradictions in logic, not noticeable by anyone.
Here, for example: "Now we know that the oxidizing agent is a substance that the electrons acquires, and the reducing agent is the substance that gives them" (Encyclopedia of the Young Chemist, an article "Oxidation and reaction)".
And immediately, two paragraphs below: "The strongest oxidizer is an electric current (a stream of negatively charged electrons)" (ibid.).
Those. the first quote states that the oxidizing agent is what electrons takes, and in the second oxidant call what gives.
And similar erroneous conclusions that contradict each other are forced to memorize in schools and institutes!
It is known that the best oxidants are non-metals. Moreover, the smaller the period number and more group number, the stronger the properties of the oxidant are expressed. This is not surprising. We disassembled the reasons for this in an article dedicated to the analysis of the periodic system, in the second part, where they spoke about the color of nucleons. From the 1st group to 8, the color of nucleons in the elements is gradually changing from purple to red (if you consider the blue color of D- and F-elements). The combination of yellow and red particles facilitates the return of accumulated free photons. Yellow accumulate, but hold weakly. And the red contribute to the return. Put photons - this is the process of oxidation. But when some are red, then there are no particles that can accumulate photons. That is why elements of 8 groups, noble gases, not oxidizing agents, unlike their neighbors, halogen.
Restoration - This is a process opposite to oxidation. Now, in science, it is believed that when the chemical element receives electrons, it is restored. This point of view is quite possible to understand (but not accept). When studying the structure of chemical elements, it was discovered that they emit electrons. Concluded that electrons are part of the elements. It means that the transfer of electrons element is, a kind, restoration of its lost structure.
However, in fact, everything is wrong.
Electrons are free photons. They are not nucleons. They are not part of the body of the element. They are attracted by entering outside, and accumulate on the surface of the nucleons and between them. But their accumulation is not at all to restore the structure of the element or molecule. In contrast, these photons emitted by them ether (energy) weaken and destroy the links between the elements. And this is the process of oxidation, but not recovery.
Restore the molecule, in reality, - take energy from her (in this case, free photons), and not report. Selecting photons, the element recoverer compacts the substance - restores it.
The best reducing agents are metals. This property naturally follows from their qualitative and quantitative composition - their fields of attraction is the largest and on the surface necessarily there are many or enough particles. of blue color.
You can even withdraw the following determination of metals.
Metal - This is a chemical element, in the composition of the surface layers of which there are necessarily blue particles.
BUT non-metal - This is an element, in the composition of the surface layers of which there is no or almost no photons of blue, and necessarily there are red.
Metals with their strong attraction perfectly take electrons. And therefore they are reducing agents.
We give the definition of the concepts of "electronegacity", "the degree of oxidation", "redox reactions", which can be found in textbooks in chemistry.
« Degree of oxidation - The conditional charge of the atom in the compound calculated on the basis of the assumption that it consists only of ions. In determining this concept, it is conventionally believed that the binders (valence) electrons go to more electronegative atoms, and therefore compounds consist of as it were from positive and negatively charged ions. The degree of oxidation may have zero, negative and positive values, which are usually set over the symbol of the element from above.
The zero value of the degree of oxidation is attributed to the atoms of elements in the free state ... The negative value of the degree of oxidation has those atoms to which the binder electron cloud (electron pair) is shifted. Fluorine in all its connections it is equal to -1. A positive degree of oxidation has atoms that give valence electrons to other atoms. For example, alkaline and alkaline earth metals, it is respectively +1 and +2. In ordinary ions, it is equal to the charge of the ion. In most compounds, the degree of oxidation of hydrogen atoms is + 1, but in the hydrides of metals (compounds with hydrogen) and others - it is equal to -1. For oxygen, the degree of oxidation -2 is characteristic, but, for example, in a compound with fluorine it will be +2, and in peroxidation connections -1. ...
The algebraic sum of the oxidation of atoms in the compound is zero, and in the complex ion - the charge of the ion. ...
The highest degree of oxidation is the greatest positive value. For most elements, it is equal to the group number in the periodic system and is an important quantitative characteristic of the element in its connections. The smallest value of the degree of oxidation of the element, which occurs in its compounds, is customary to be called a lower degree of oxidation; All other - intermediate "(Encyclopedic Dictionary of the Young Chemist, article" Degree of oxidation ").
Here are the basic information relating to this concept. It is closely related to another term - "Electricity".
« Electricity - This is the ability of an atom in the molecule to attract electrons involved in the formation of a chemical bond "(Encyclopedic Dictionary of the Youth Chemist, article" Electricity ").
"Redox reactions are accompanied by a change in the degree of oxidation of atoms that are part of the reacting substances, as a result of moving electrons from the atom of one of the reagents (reducing agent) to the other atom. With oxidative and reducing reactions, oxidation (electron return) and recovery (electron addition) occur (chemical encyclopedic dictionary) (chemical encyclopedic dictionary Ed. I.L. Knunyantz, article "Oxidation and reducing reactions").
In our opinion, there are quite a few mistakes in these three concepts.
Firstly , we believe that the formation of a chemical connection between the two elements is not at all the process of the socialization of their electrons. Chemical bond is a gravitational connection. Electrons, allegedly flying around the kernel, are free photons accumulating on the surface of the nucleons in the body of the element and between them. In order for between two elements a connection arose, their free photons do not need to run between the elements. This does not happen. In fact, the heavier element takes off (attracts) free photons with lighter, and leaves them (more precisely, on yourself). And the zone of a lighter element with which these photons were removed, to one way or another. Because of which attraction in this zone is manifested to a greater extent. And the easier element is attracted to heavily. So the chemical bond arises.
Secondly , Modern chemistry sees the ability of the elements to attract electrons distorted - inverted. It is believed that the larger the electronegativity of the element, the more he is able to attract electrons to itself. And the fluorine with oxygen allegedly makes it best - they attract other electrons to themselves. As well as other elements 6 and 7 groups.
In fact, this opinion is no more than a delusion. It is based on the erroneous view, as if the more group number, the harder items. And also, the greater the positive charge of the nucleus. This is bullshit. Scientists do not even disturb themselves to explain that from their point of view is a "charge." Simply, as in Numerology, they recalculated all the elements in order, and were signed in accordance with the number of charge values. Great hike!
This is clear and child that gas is lighter than dense metal. How did it happen that in chemistry it is considered that the gases are better attracting electrons?
Dense metals, of course, they are better attracting electrons.
Scientists-chemists, of course, can leave the concept of "electronegance" in the course, since it is so common. However, they will have to change its meaning on the opposite direct.
Electricity - This is the ability of the chemical element in the molecule to attract electrons. And, of course, at metals, this ability is better expressed than non-metals.
As for the electric poles in the molecule, then, indeed, negative pole - These are non-metal elements that give electrons with smaller fields of attraction. BUT positive - It is always elements with more pronounced metallic properties, with large fields of attraction.
Smile together.
Electricity - This is another one, another attempt to describe the quality of the chemical element, along with already existing weighing and charge. As it often happens, scientists from a different area of \u200b\u200bscience, in this case, chemistry, as if not trusting with their colleagues to physicists, but rather, simply because any person, making discoveries, is in its own way, and not just exploring the experience of others.
So it happened this time.
The mass and charge did not help any chemists to understand what was happening in atoms when they were interacting with each other - and electronegate was introduced - the element's ability to attract electrons involved in the formation of a chemical bond. It should be recognized that the idea of \u200b\u200bthis concept is laid very true. With the only amendment that it reflects the reality in an inverted form. As we said, it is best to attract metal electrons, rather than non-metals - by virtue of the characteristics of the color of surface nucleons. Metals are the best reducing agents. Nonmetals - oxidizing agents. Metals are taken, non-metals give. Metals - Yin, Nemmetalla - Yang.
Esoteric comes to help science in matters of comprehending the secrets of nature.
Concerning oxidation degree This is a good attempt to understand how the distribution of free electrons is occurring within the chemical compound - molecules.
If the chemical compound is uniformly - that is, it is simple, its structure consists of elements of the same type - then everything is true, really the degree of oxidation of any element in the compound is zero. Since there are no oxidants in this connection and no rebooters. And all the elements are equal in quality. No one takes the electrons, no one gives. Whether it is a dense substance, or liquid, or gas - no matter.
The degree of oxidation, as well as electronegativity, demonstrates the quality of the chemical element - only within the framework of the chemical element. The degree of oxidation is designed to compare the quality of chemical elements in the compound. In our opinion, the idea is good, but its implementation does not quite satisfy.
We categorically against the whole theory and concept of the structure of chemical elements and connections between them. Well, if only because the number of groups, according to our ideas, should be more than 8. And therefore, the whole system collapses. Yes, and not only that. In general, recalculate the number of electrons in the "fingers" atoms is somehow not serious.
In accordance with the current concept, it turns out that the strongest oxidizers are assigned the smallest conditional charges - fluorine has a charge -1 in all compounds, oxygen almost everywhere -2. And in very active metals - alkaline and alkaline earth - these charges, respectively, +1 and +2. After all, it is absolutely not logical. Although, we repeat, we understand the general scheme very well, in accordance with which it was done - all for the sake of 8 groups in the table and 8 electrons in the external energy level.
However, at a minimum, the magnitude of these charges in halogen and oxygen was supposed to be the greatest with a minus sign. And alkaline and alkaline earth metals are also big, only with a plus sign.
In any chemical compound There are elements that give electrons - oxidizing agents, non-metals, negative charges, and elements that take electrons - reducing agents, metals, positive charge. This is exactly the way to compare the elements, relate them to each other and try, determining their degree of oxidation.
However, to find out in this way the degree of oxidation, in our opinion, does not quite accurately reflect the reality. It would be more correct to compare the electronegability of the elements in the molecule. After all, electronegativity is almost the same as the degree of oxidation (characterizes the quality, only a separate element).
You can take the electricity scale and put it in the formula for each element. And then it will immediately be seen, which elements are given electrons, and which are taken. That element whose electronegativity in the compound is the highest - negative pole, gives electrons. And the one whose electronegability is the smallest - positive pole, takes the electrons.
If items, admit, 3 or 4 in the molecule, nothing changes. All also put the magnitude of electronegability and compare.
Although it should not be forgotten to draw the model of the structure of the molecule. Indeed, in any compound, if it is not simple, that is, it does not consist of one type of elements, are associated with each other, first of all, metals and non-metals. Metals select electrons in non-metals, and bind to them. And in one element of non-metal, electrons 2 can be selected at the same time or a larger number of elements with more pronounced metal properties. So the complex, complex molecule occurs. But this does not mean that in such a molecule, metal elements will come in solid connection and with each other. Perhaps they will be located on opposite sides from each other. If nearby - they will attract. But the solid connection is formed only if one element is more metallic than the other. Be sure to make one element select the electrons - removed. Otherwise, the element will not happen - exemption from free photons on the surface. The attraction field will not manifest well, and there will be no durable connection. it complex topic - Education chemical tiesAnd we will not tell about it in detail in this article.
We believe, we highly lit the topic dedicated to the analysis of the concepts of "electronegacity", "oxidation degree", "oxidation" and "restoration", and provided to your attention a lot of curious information.
From book managed dreams the author of the world ElenaRestoration "When an unified sign of Indi-Appeam is born, essence and life is divided into two. From this point on, if the final world does not reach, the essence and life will never see each other again. " William, "Mystery of the Golden Flower" after the Institute
From the book the book of secrets. Incredible obvious on earth and abroad Author Vyatkin Arkady DmitrievichMasochism as an extreme degree of voluntary vampirism in this sense Masochism is similar to telecommunication. Mazochists are people who receive pleasant sensations from their own physical and mental suffering. In other words, they like it when they beat, scold, mock
From the book Vampires in Russia. All you need to know about them! Author Bauer AlexanderHow to determine the degree of blood loss when the vampire drinks blood, then drinks at once from the half-liter to one and a half liters of blood. The human body contains only five or six liters of blood, so that such blood loss is not necessarily dangerous for life. However, the vampire can
From the book modeling the future in a dream the author of the world ElenaRestoration after the institute distribution, working as an engineer in a closed company, I realized that I was not in my place, so I decided to change the profession and entered the jazz school of improvisation, and later in the music school for the classic office.
From the book Golden Rules Fengshui. 10 simple steps to success, well-being and longevity Author Rudin Valentin LeonidovichThe degree of negative impact of external objects The greatest negative influence external objects have, being immediately before entering the house. But the more they are located at an angle to the entrance, the weaker becomes their influence. The object is directly
by the author Eduard CureFirst degree: cooking. The Nagorny Sermon and the Kingdom of God's case of Christ begins with the Galilee idyll and the announcement of the "kingdom of God." This prediction indicates us to its popular instructions. At the same time, it is a preparation for more elevated
From the book Divine Evolution. From sphinx to Christ by the author Eduard CureSecond degree of initiation (cleansing). Wonderful healing. Christian therapy in all ancient mysteries for moral and intellectual preparation should have been cleansing the soul, which should revive new organs in it and give it subsequently the ability
From the book a big sphinx riddle author Barbaren GeorgesRestoration of the statue The actual age of a large sphinx goes back to the beginning of the Adam era. At least he is a contemporary pyramid, the ensemble of which he, as we will see, completed it. The image of a large Sphynx has been subjected for expired centuries
From the book Healing of the Soul. 100 meditative techniques, healing exercises and relaxation Author Rajnish Bhagwan SriRestoration of the rhythm ... Install the same time to go to bed - if every evening it is eleven, it means that eleven. This is the first: get a certain time, and soon the body will be able to enter this rhythm. Do not change this time, otherwise you will choose the body. Body
by the author Kuzmishin E. L.Degree of a student Reception to the student's degree. The decoration of the lodge and the domains and the ceiling of the lodge should be baked blue and white matter without gilding. Over the head of the expert master is located surrounded by a shine of a triangle with the name in his center
From the book of Caliosostro and Egyptian Freemasonry by the author Kuzmishin E. L.Reception in the student's degree. The decoration of the lodges and the domains and the ceiling of the lodge should be hung with blue and white matter without gilding. Over the head of the expert master is located surrounded by a shine of a triangle with the name "Jehovah", embroidered in his center
From the book Autobiography Yoga Author Yogananda ParamyansaChapter 23 I get a university degree - you ignore the philosophical definitions from the textbook undoubtedly counting on that some non-rounding "intuition" will hold you through all exams. But if you urgently do not consult a more scientific method, then I will have to
From the book of Kabbalah. The highest world. The beginning of the way Author Lightman Michael.7.5. The degree of awareness of evil as clarified in the article "Torusting", pleasure and bliss are determined by the degree of similarity of the Creator according to the properties, and suffering and impatience - the degree of differences from the Creator. In accordance with this, egoism is disgusting to us and unbearably paint,
Option 1
1. Determine the degree of oxidation of atoms of chemical elements in the following connections: HNO₃, NO₂, H₃N, SO₂, N₂O.
2. Taking advantage of the periodic table, position the following elements in order to reduce electronegativity: O, N, BE, B, LI, C. Determine where fluorine and sodium should be located in this row. Explain the answer. 
3. How does the degree of oxidation of the sulfur atom change when SO₂ to SO₃? Reply explain the answer.
Option 2.
1. Determine the degree of oxidation of atoms of chemical elements in the following connections: CO₂, H₃PO₄, SIH₄, P₂O₅, MG₂SI.
2. In the direction of atoms of which chemical element are shifted by general electronic couples, Molecules of the following connections: BF₃, PCL₃, CS₂, CCL₄, HBr? Give a reasonable answer.
3. Whether the degree of carbon oxidation changes during formation coalic acid H₂CO₃ carbon dioxide and water? Reply explain the answer.
Option 3.
1. Determine the degree of oxidation of atoms of chemical elements in the following connections: CL₂, NACLO, CACL₂, HF, SO₃, CL₂O₇.
2. Using periodic system Chemical elements, position the following elements in the order of increasing their electronegability: p, al, cl, na, s, mg. Find a potassium and fluorine in this row. Reply explain the answer. 
3. How does the degree of carbon oxidation change when burning methane CH₄ with the formation of carbon oxide (IV) and water? Reply explain the answer.
Option 4.
1. Determine the degree of oxidation of atoms of chemical elements in the following connections: H₂SO₄, SO₂, NO₂, BF₃, H₂S.
2. towards the atoms of which chemical elements Displays general electronic pairs, in the molecules of the following connections: H₂O, Pcl₃, H₃n, H₂S, CO₂? Give a reasonable answer.
3. Whether the degrees of oxidation of atoms are changed when water is changed from simple substances - hydrogen and oxygen? Reply explain the answer.


